Custom Buffer and Reagent Manufacturing and Filling Service
Full-Service Reagent Support—Beyond Component Manufacturing
At Ciro Manufacturing, we offer more than precision component manufacturing. Our capabilities extend to reagent formulation, testing, filling, labeling, inspection, and shipping—providing a complete solution for your life science and diagnostic products.
Reagent Formulation
We can formulate aqueous-phase reagents in volumes ranging from a few hundred milliliters to several hundred liters. Your team specifies the exact composition—buffer systems, detergents, preservatives—and we’ll prepare it to your specifications. All formulations are produced in cleanroom environments (ISO 5, 6, or 7), depending on your application. Particulate filtration is available as required.
Quality Testing
We perform testing based on your standard operating procedures. All QC personnel undergo extensive, project-specific training to ensure accuracy and consistency. Our sample preparation and testing areas are physically separated to prevent cross-contamination and maintain the integrity of your test results.
Filling Capabilities
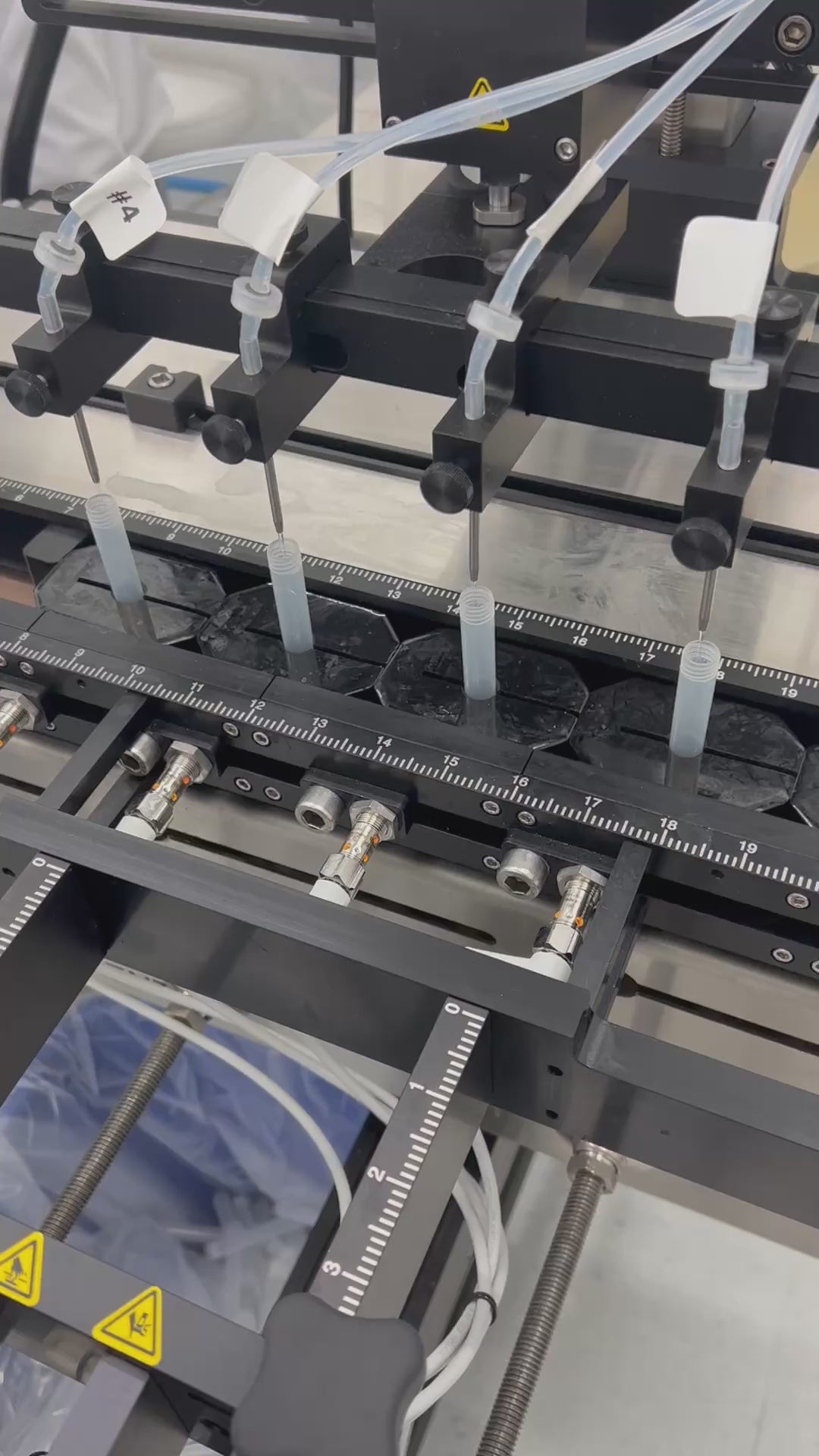
Ciro supports a wide range of filling formats using both automated and manual systems:
- in cleanrooms for high-volume production
- using micropipettes, repeaters, and peristaltic pumps under ISO 5 laminar flow hoods
- microliters to multiple liters
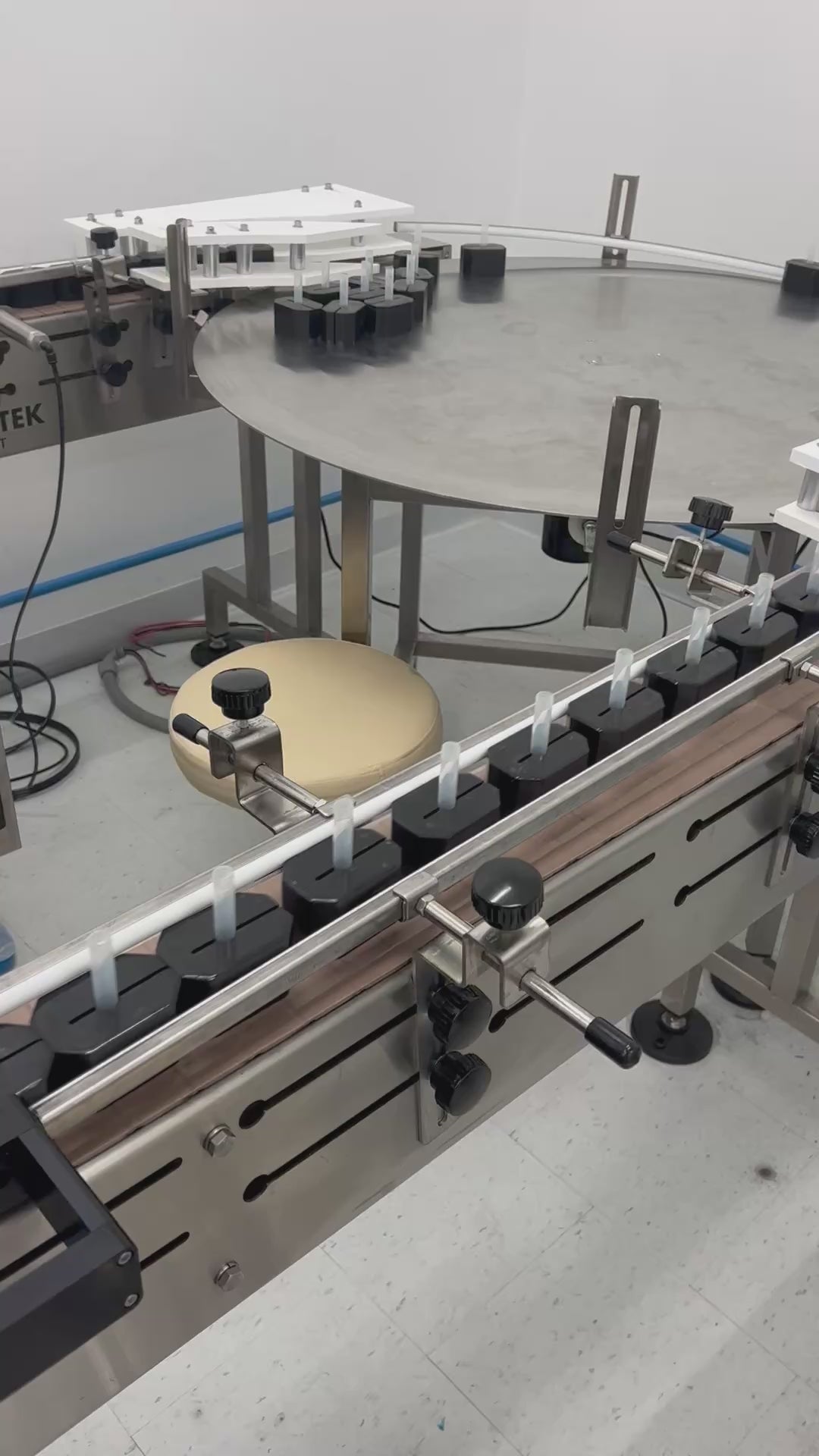
Whether you're launching a new assay kit or scaling an existing formulation, our team ensures your reagents are processed, filled, and packaged with precision and care.
Labeling, Label Control & Inspection—Precision and Compliance You Can Trust
At Ciro Manufacturing, labeling is more than just a finishing step—it’s a critical part of product integrity and regulatory compliance. We apply strict controls and quality assurance measures at every stage of the labeling process, from initial print setup to final vial inspection.
Whether you require custom-branded packaging, UDI barcodes, or regulatory-compliant labeling for FDA or international markets, Ciro ensures accurate, traceable, and professional results.
Full-Service Labeling & Quality Assurance
- Application by Trained Operators
All labeling is performed by qualified personnel using approved SOPs and validated methods for positioning, adhesion, and alignment. - Label Reconciliation and Waste Management
Unused or excess labels are fully tracked and securely destroyed. Our label reconciliation process prevents labeling mix-ups and maintains compliance with ISO 13485 and FDA 21 CFR Part 820 requirements. - Custom & Regulatory Labeling
We support everything from clinical trial identifiers and batch barcodes to CE-marked layouts, multilingual IFUs, and private labeling.
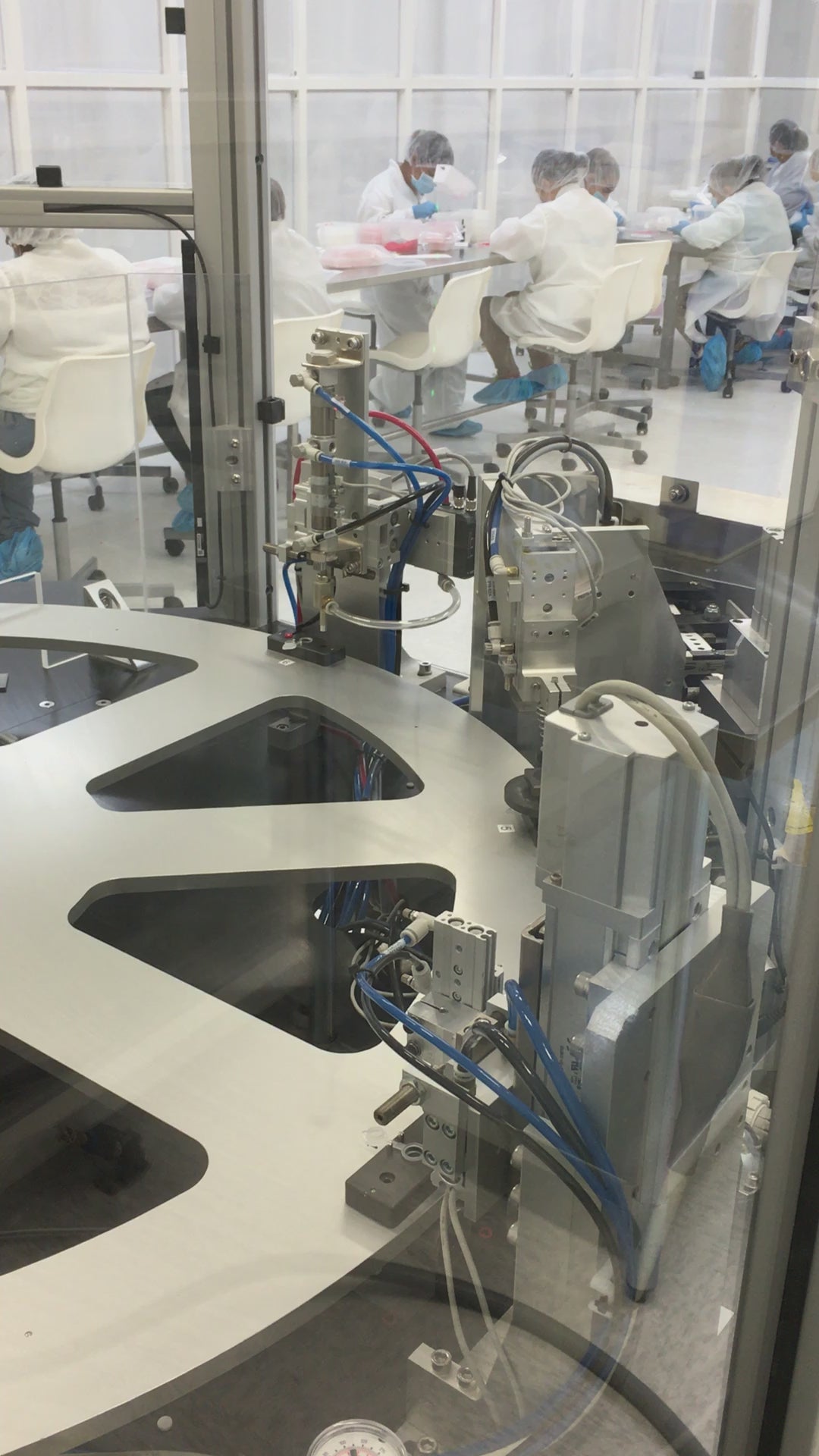
In-Line & Post-Labeling Inspection
Every unit undergoes visual and functional inspection for both critical and non-critical defects, including:
- Label alignment and adhesion
- Correct label content and barcode scannability
- Damage-free surface and clarity
- Legibility and print quality
Inspection results are recorded and retained for full traceability and audit readiness.
Ensure every unit that leaves your facility is correctly labeled, fully compliant, and market-ready. Let Ciro’s meticulous label control and inspection processes safeguard your product and brand.