Cleanroom Injection Molding—Contamination-Free Precision for Medical and Life Science Products
In the medical, biotech, and diagnostics industries, product performance can hinge on one invisible factor: contamination. That’s why cleanroom injection molding has become essential for manufacturers requiring sterile, particulate-controlled production environments.
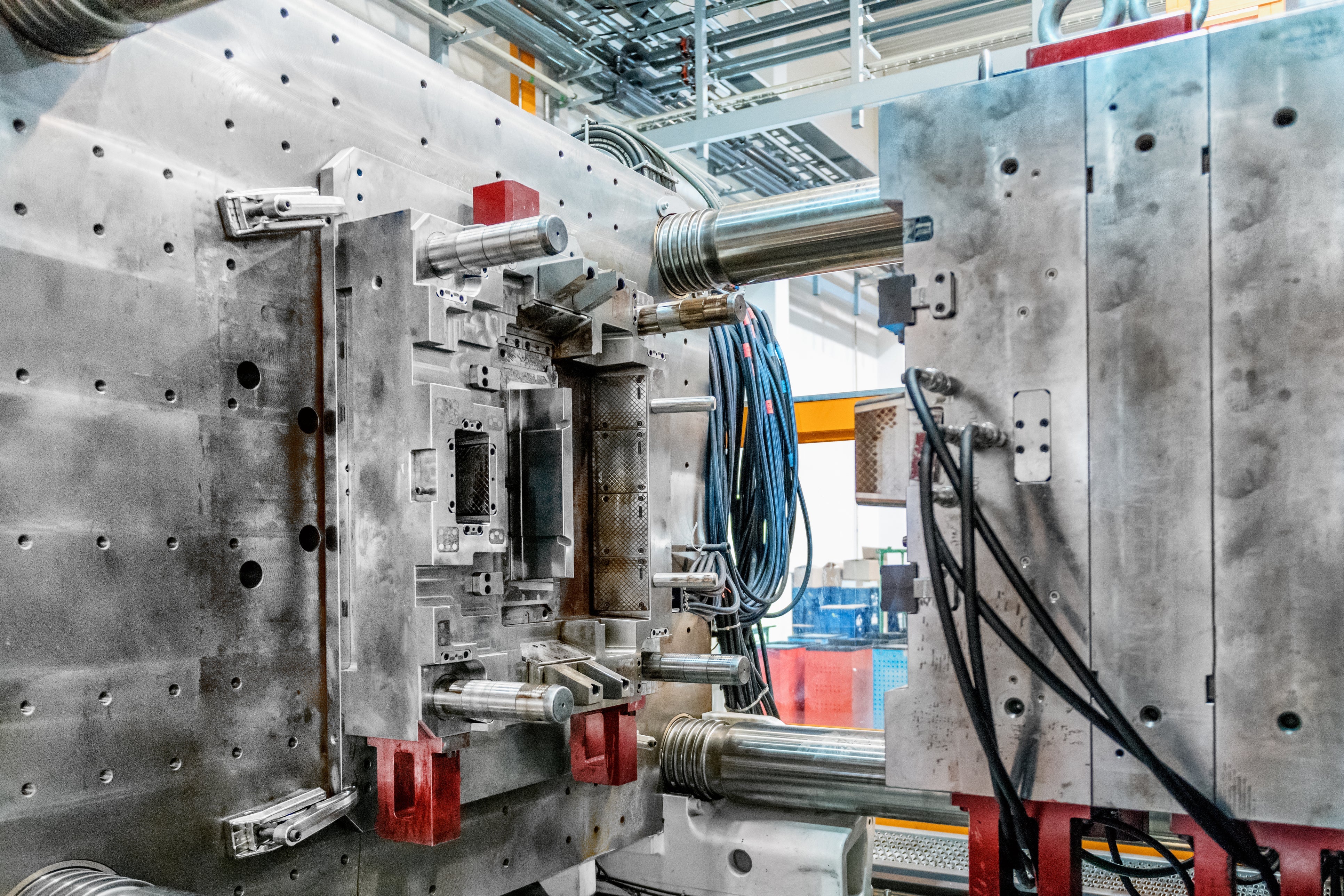
At Ciro Manufacturing, we offer ISO Class 6 cleanroom injection molding to produce high-precision, medical-grade plastic components under ISO 13485-certified systems. From swab handles and diagnostic housings to sample tubes and reagent reservoirs, we mold parts that support both patient safety and regulatory compliance.
What Is Cleanroom Injection Molding?
Cleanroom injection molding combines the technical rigor of traditional plastic molding with the environmental control of an ISO-certified cleanroom. The result is a part produced under tightly regulated temperature, humidity, and air particulate standards—free from dust, debris, or microbial contamination.
This process is ideal for:
-
Single-use diagnostic components
-
Medical device parts that contact the patient or sample
-
Sterile labware and biotech consumables
-
Packaging components for sensitive reagents
Our Cleanroom Molding Capabilities
1. ISO Class 6 Cleanroom Environment
Our molding operations are housed within a validated ISO Class 6 cleanroom, equipped with:
-
HEPA filtration and air exchange systems
-
Particle count monitoring
-
Gowning and personnel access protocols
2. Medical-Grade Resin Handling
We process a wide range of FDA and USP Class VI polymers, including:
-
Polypropylene (PP)
-
Polycarbonate (PC)
-
COC/COP
-
ABS
-
Thermoplastic elastomers (TPEs)
All materials are traceable from batch to final part and stored in contamination-controlled systems.
3. Precision Multi-Cavity Molds
We design and operate custom tools with:
-
Tight tolerance control
-
Balanced runners and optimized fill
-
Insert and overmold capabilities
-
Mold validation and IQ/OQ documentation
4. In-Line Inspection and Cleanroom Packaging
Each production run includes:
-
Visual and dimensional QC checks
-
Lot-level documentation
-
Cleanroom bagging and labeling for downstream sterile processing
Why Choose Ciro for Cleanroom Molding?
-
Speed + Control: In-house tooling, molding, and cleanroom assembly eliminate delays and handoffs
-
Compliance Built In: ISO 13485 QMS supports FDA, CE, and IVDR regulations
-
Scalability: We support pilot runs and ramp to high-volume production
-
Quality Assurance: Every batch includes DHRs, CoCs, and traceable production records
Applications We Serve
-
PCR and lateral flow test components
-
Reagent tubes and closures
-
VTM transport media containers
-
Surgical and procedural tool components
-
Biotech liquid handling consumables
Case Study: Sterile Swab Handle Production for Diagnostic Kits
During a major public health initiative, a client needed millions of molded swab handles in a Class 6 cleanroom. Ciro:
-
Designed and fabricated the mold
-
Ran 24/7 molding in our cleanroom
-
Delivered validated, contamination-free parts with lot tracking
Outcome: Product passed all sterility and regulatory requirements for nationwide use.
Final Thought: Molded for Safety and Compliance
At Ciro Manufacturing, cleanroom injection molding is more than a service—it’s a safeguard. Whether you’re protecting patient health or ensuring test accuracy, our cleanroom processes give your product the purity it needs to perform and succeed.
👉 Let’s build your next cleanroom-ready solution together.